Abstract
Introduction:
Chronic myeloid leukemia (CML) is characterized by the presence of the BCR-ABL1 translocation and is typically distinct from other myeloproliferative neoplasms (MPN) including polycythemia vera (PV), essential thrombocythemia (ET) and myelofibrosis (MF). Concurrent diagnosis of CML and MF is uncommon and infrequently described in case reports.1 Moreover, very few cases have been described of CML occurring after an established diagnosis of MPN.2-3 Due to the rarity of concurrent CML and MPN, management strategies in this population are not defined. In addition, the latency time from the emergence of BCR-ABL1 positive clones to a clinical diagnosis of CML in this setting is largely unknown. To address these questions, we interrogated our institutional experience and retrospectively analyzed available specimens.
Methods:
We collected information on patients seen at Massachusetts General Hospital (MGH) from January 1 2012 to January 1 2022 for CML occurring after an established diagnosis of MPN. Available stored samples were retrospectively tested for BCR-ABL1 with a fusion transcript assay (MGH Center for Integrated Diagnostics, Boston, MA).
Results:
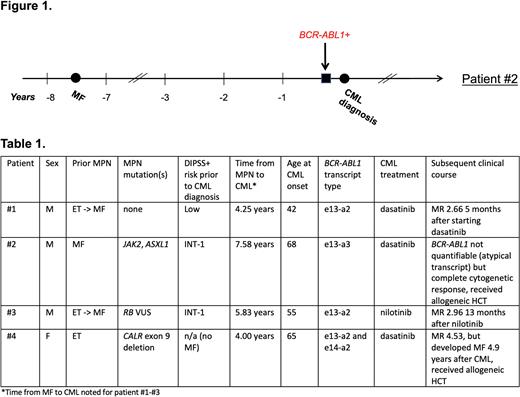
We identified four patients with CML preceded by MPN (Table 1). Three patients had prior MF, diagnosed 4.3-7.6 years before the onset of CML; two of these had post-ET MF. Patient #4 had ET diagnosed 4 years prior to onset of CML, then went on to develop MF 4.9 years after CML diagnosis. None of the patients were on JAK inhibitors, hydroxyurea or interferon at the time of CML diagnosis. All patients had a leukocytosis with characteristic features on the differential prompting evaluation for CML. The median white blood cell count was 45.83 x 10^9/L (range 27.49-121.02). All four patients had an elevated basophil count (median 2.99 x 10^9/L, range 1.1-22.99) and two had an elevated eosinophil count (median 1.11 x 10^9/L, range 0.55-44.17). Regarding MPN driver mutations, one patient had a JAK2 V617F, one patient had a CALR exon 9 deletion, and two patients had no identifiable mutations on extended next-generation sequencing.
For CML treatment, three patients received dasatinib (two with 100 mg daily, one with 50 mg daily), whereas one received nilotinib (150 mg twice daily). Two achieved a major molecular response (MMR) (at 7 and 8 months); one had an early molecular response at 6 months (last follow-up); one (with e13-a3 transcript) could not be quantified on a standard PCR assay but achieved a complete cytogenetic response by 4 months. At the time of last follow-up, two patients were in MMR on tyrosine kinase inhibitors (TKI), whereas two underwent allogeneic hematopoietic cell transplantation (alloHCT), primarily for progression of MF.
We retrospectively interrogated available samples for evidence of the BCR-ABL1 translocation prior to a clinical diagnosis of CML. Patient #2 was followed at MGH for MF and thus had available samples. We identified BCR-ABL1 transcripts in the peripheral blood three months prior to his clinical diagnosis of CML (unable to be quantified due to e13-a3 transcript); there were no available samples from earlier in his disease course for testing (Figure 1).
Discussion:
CML can emerge in patients with MPN. Herein, we describe four cases of this phenomenon. Signs of CML evolution included marked leukocytosis, as well as basophilia and eosinophilia. Despite underlying MF, three patients tolerated full-dose TKI and achieved molecular responses early in their disease course. However, MPN progression remained a significant challenge, with two patients ultimately requiring an alloHCT. Of note, we found evidence of BCR-ABL1 in the peripheral blood three months prior to clinically apparent CML in one patient. Experiments using single cell techniques in patients with concurrent MPN and CML would provide insights into the clonal architecture and are in planning stages.
References:
1. Bornhäuser M, et al. Concurrent JAK2(V617F) mutation and BCR-ABL translocation within committed myeloid progenitors in myelofibrosis. Leukemia 2007; 21(8): 1824-1826.
2. Yamada O et al. Emergence of a BCR-ABL translocation in a patient with the JAK2V617F mutation: evidence for secondary acquisition of BCR-ABL in the JAK2V617F clone. J Clin Oncol 2014; 32(21): e76-79.
3. Jallades L, et al. Emergence of therapy-unrelated CML on a background of BCR-ABL-negative JAK2V617F-positive chronic idiopathic myelofibrosis. Leuk Res 2008; 32(10): 1608-1610.
Disclosures
Fathi:Takeda: Consultancy; Genentech: Consultancy; Ipsen: Consultancy; Forma: Consultancy; Celgene/BMS: Consultancy, Other: Clinical Trial Support; Amgen: Consultancy; Novartis: Consultancy; Abbvie/Servier: Consultancy, Other: Clinical Trial Support; Astellas: Consultancy; Kite: Consultancy; Foghorn: Consultancy; Morphosys: Consultancy; Mablytics: Consultancy; Immunogen: Consultancy; Orum: Consultancy; EnClear: Consultancy; PureTech: Consultancy; AbbVie, Agios, Bristol Myers Squibb, Servier, and Takeda: Research Funding; AbbVie, Agios, Amgen, Astellas Pharma, Blueprint Medicines, Bristol Myers Squibb, Daiichi Sankyo, Foghorn Therapeutics, Forty Seven, Inc., Genentech, Ipsen, Kite Pharma, Kura Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; MorphoSys, Novartis, Pfizer, Seattle Genetics, Takeda, Trillium Therapeutics, and Trovagene: Consultancy, Membership on an entity's Board of Directors or advisory committees. Hobbs:Incyte: Other: Advisor or review panel participant; PI, Research Funding; Abbvie Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant; Constellation: Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant; PI, Research Funding; Pfizer: Other: Advisor or review panel participant; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant; Pharmaxis: Other: Advisor or review panel participant; Keros: Other: Advisor or review panel participant; Bristol Myers Squibb Co./Celgene: Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant; Bayer: Research Funding; Merck: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.